西北高原生物研究所研究发现重金属矿物药中硫化汞溶出新规律
发表日期:2018-04-16来源:西北高原生物研究所
放大缩小
西北高原生物研究所藏药药理与安全性评价研究学科组(魏立新研究组)近期研究发现,肠道中L-半胱氨酸对中药朱砂和藏药佐太中主要汞化合物——硫化汞中汞的溶出具有pH依赖的促进作用。α-HgS和β-HgS分别是含汞传统药物朱砂和佐太中汞的主要存在形式。朱砂和佐太由于含有大量的汞化合物,其临床安全性受到广泛质疑。硫化汞在胃肠道中溶出机制是朱砂和佐太临床安全性研究中亟需回答首要问题。L-半胱氨酸是唯一含有自由巯基的常见氨基酸,在汞的肠道溶出和吸收中发挥重要作用。
该研究表明,在胃肠道pH1.2~7.2范围内,β-HgS在含L-半胱氨酸的水溶液中的汞溶出量随pH值上升而增加;但是,α-HgS汞溶出量随pH值上升呈“增加——减少——增加”的规律。这种汞溶出规律的差异导致,在肠道pH范围内(pH4.8~7.2),β-HgS的汞溶出量是α-HgS的114.3到365.5倍,远高于两者在溶度积常数上4倍的差异。
该研究成果对以中药朱砂和藏药佐太为代表的含汞传统药物的质量控制、安全服用方法及其汞的肠道吸收研究具有重要的理论意义和应用价值。
该研究结果于2018年1月29日发表在Biological Trace Element Research杂志上(Zhang Ming, Bi Hongtao*, Li Cen, Du Yuzhi, Wei Lixin*. pH-Dependent Effects of L-Cysteine on Mercury Dissolution of α-HgS and β-HgS. Biol Trace Elem Res (2018). https://doi.org/10.1007/s12011-018-1254-9)。
(责任编辑:西北研究院 陈治理)
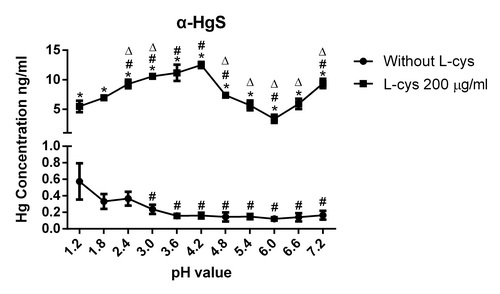
Fig. 1. Effects of L-cysteine on mercury dissolution of α-HgS in pH1.2-7.2 buffurs
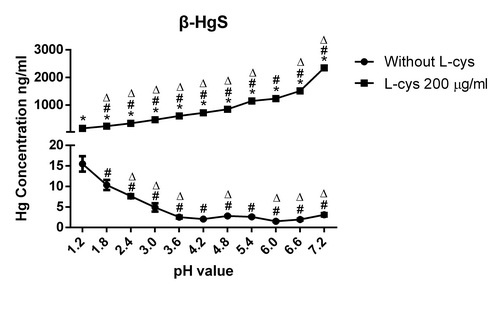
Fig. 2. Effects of L-cysteine on mercury dissolution of β-HgS in pH1.2-7.2 buffurs
ABSTRACT:Mercury sulfide is an insoluble inorganic mercury compound, and it is the main chemical form in traditional oral mercury-containing medicines. Hg2+ has a high affinity for thiols, and small molecule thiols in the gastrointestinal tract may promote mercury dissolution of mercury sulfide by binding to Hg2+. L-cysteine is the only amino acid that possesses a reducing sulfhydryl group (-SH), out of the 20 amino acids. This study investigates the effect of L-cysteine on mercury dissolution of mercury sulfide at pHs ranging from 1.2 to 7.2. The results showed that L-cysteine had different pH-dependent effects on the mercury dissolution of α-HgS and β-HgS. For α-HgS, the dissolved mercury concentration increased from 5.47?±?0.97 ng/mL to 12.49?±?0.54 ng/mL when the pH rose from 1.2 to 4.2, and decreased to 3.37?±?0.70 ng/mL at pH 6.0 and then increased to 9.36?±?0.79 ng/mL at pH 7.2. For β-HgS, the dissolved mercury concentration increased from 151.09?±?2.25 ng/mL to 2346.71?±?62.62 ng/mL when the pH increased from 1.2 to 7.2. In conclusion, L-Cys was distinctly enhanced upon mercury dissolution of α-HgS and β-HgS with increasing pH. These results may contribute to our understanding of the mercury absorption mechanism of traditional oral mercury-containing medicines.
附件
扫一扫在手机浏览








